RICOH launches RICOH Standard DNA Series of reference DNA plates that overcome challenges in PCR testing
TOKYO, April 15, 2020 – Ricoh Company, Ltd. will begin marketing its newly developed the RICOH Standard DNA Series as a reference material*2 for genetic testing*1 applications where PCR is used.
The RICOH Standard DNA Series uses Ricoh's proprietary bioprinting technology to enable a specific number of DNA molecules, in units of one, to be injected into containers used for genetic testing. This means that the accuracy of detection in PCR tests can be assured even in low concentrations of under 100 molecules.
Previously Ricoh supplied reference DNA plates for noroviruses, but it has now expanded the use of this product by developing reference DNA plates for specific types of viruses, including novel coronavirus (SARS-CoV-2). Currently, these are only available in Japan.
 RICOH Standard DNA Series for Noroviruses
RICOH Standard DNA Series for Noroviruses
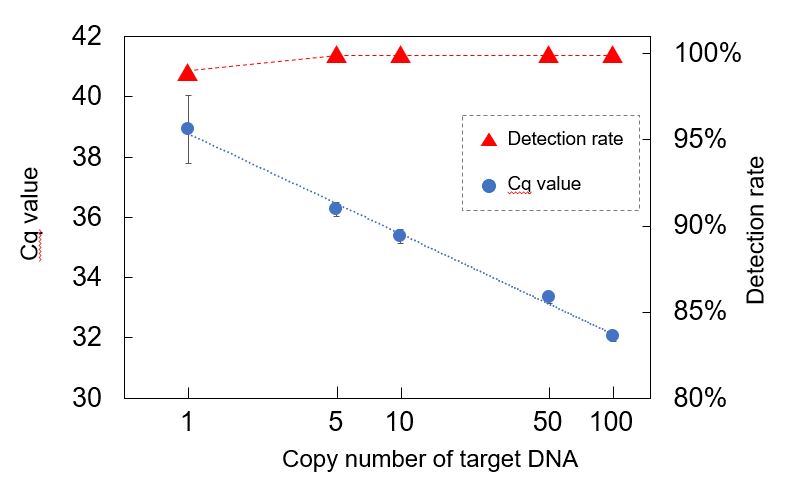 Evaluation of reference DNA plate for the novel coronavirus (SARS-CoV-2) by real-time PCR*3
Evaluation of reference DNA plate for the novel coronavirus (SARS-CoV-2) by real-time PCR*3 In principle, PCR is a high-performance genetic testing method that can detect DNA even at the level of a single molecule by amplifying it. In reality, extremely small amounts of DNA cannot be detected in some tests because of inadequate precision control in the apparatus or imperfect performance and quality of reagents. Therefore, “false negatives” occur, where viruses cannot be detected even though the person is infected, and this makes accurate diagnosis of viral diseases challenging.
The accuracy of PCR tests is determined by their sensitivity and specificity. Sensitivity refers to the proportion of cases in which people infected with the virus (true positives) are correctly identified as “positive”. Specificity refers to the proportion of cases in which people who are not infected with the virus (true negatives) are correctly identified as "negative." One of the reasons for inaccurate detection may be insufficient verification of the sensitivity and specificity of the PCR test. In addition, there are detection limits with PCR testing when trace amounts of a virus below a certain limit cannot be detected. In the event a sample has a viral level below the detection limit at the time of test, the result of the PCR test will be “negative,” even if the virus is present in the sample, and consequently the result will be a “false negative.” “False negatives” can lead to new infections because patients carry out daily activities without realizing they are infected. Accordingly, reducing “false negatives” can contribute to reducing the risk of the infection spreading.
To verify the detection limit and the sensitivity of PCR testing, and to accurately measure and control the performance and quality of test instruments and reagents, it is necessary to use reference material where the number of DNA molecules is specified accurately as a standard in the test. Reference materials for genetic tests have already been supplied by several companies and research institutes, but they are highly concentrated materials with the number of DNA molecules specified in moles (the number of molecules per mole is 6.02 × 1023). Although there is no problem with high-concentrations, when this reference material is diluted to create a standard with various numbers of DNA molecules, it has been difficult with low-concentrations of less than 100 molecules to judge whether or not the measurement is accurate because variations occur in the number of DNA molecules.
The RICOH Standard DNA Series solves this problem in PCR testing. This product uses bioprinting technology with a unique inkjet method to dispense DNA molecules into the wells of plates or tubes for genetic testing in units of one molecule. This means that there is no variation in the number of DNA molecules, even at low concentrations, and it makes it possible to exercise strict precision control and quality control over genetic testing methods, testing devices, and reagents, etc. The number of DNA molecules injected per well can also be increased incrementally as required.
In the future, Ricoh will expand the applications of the RICOH Standard DNA Series to precision control of genetic testing methods and reagents, to handle unknown infectious diseases and contribute to virus free tests (tests that certify the absence of viruses) used in the manufacture of regenerative medicines and biopharmaceuticals, etc.
※This product is a reagent for research use only.
Relevant Information
(News Release) Bioprinting Technology to Control the Number of DNA Molecules in Units of On
https://www.ricoh.com/release/2018/0604_1.html
(Technical page) The Manufacturing of reference DNA plates
https://www.ricoh.com/technology/institute/research/tech_dna_reference_plate.html
Biomedical Business Center
Healthcare Business Group
E-mail: [email protected]
Public Relations
E-mail: [email protected]
News & Events
Keep up to date
-
04 Feb
Ricoh and Global Vision Multimedia Launch Strategic Partnership Negotiation in the Asia-Pacific region
-
02 Feb
Ricoh named a Clarivate Top 100 Global Innovator 2026
-
27 Jan
Ricoh named in the 2026 Global 100 Most Sustainable Corporations by Corporate Knights
-
14 Jan
Ricoh named to CDP’s double A List for the third consecutive year
